Produced by
Country of origin
The Republic of Korea

Detects
Antigen

Biomaterial
Saliva
Philosys Gmate COVID-19 Ag Saliva
99%
Specificity
100%
Accuracy
95%
Sensitivity

15 minutes - ready time
Helps in confirming the diagnosis
Easy to use, no laboratory equipment required
Rapid test for the presence of coronavirus antigen
Product overview
1 / 7
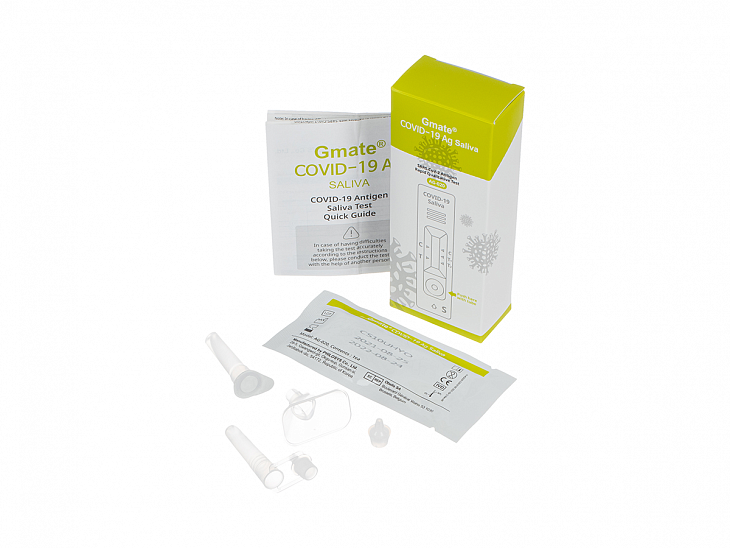
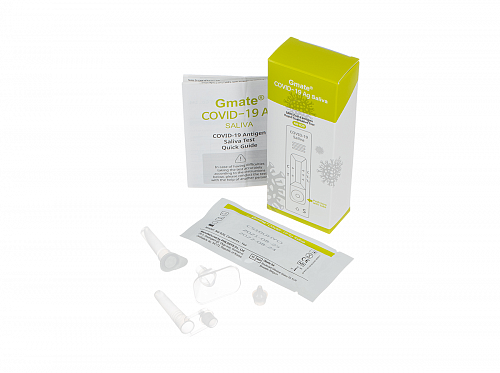
A set of reagents for lateral flow testing of human saliva samples for SARS-CoV-2 antigen (Gmate COVID-19 Ag Saliva). Lot CS10UHYO.
The set is used as a preliminary assessment for diagnosing novel coronavirus infections (COVID-19). When testing biomaterial samples with this kit, results that confirm the reactivity of the sample should be confirmed by supplemental analysis.
The product contains a set of reagents, including:
-
Test cassette — 1 pc.;
-
Test tube with buffer and nozzle with dropper — 1 pc.;
-
Tube for collecting sample and a cap with dropper and funnel for collecting saliva — 1 pc.;
-
Usage instructions — 1 pc.
Use order
Biomaterial sampling
Adding 10 μL of sample to the well
Expect the result according to the time in the instructions
Interpreting the rapid test results
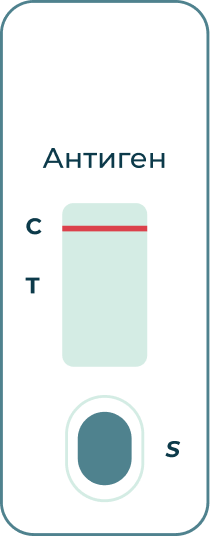
If the (C) line turns red:
The test returned a negative result for the presence of coronavirus antigen.
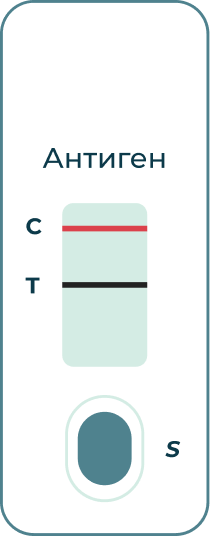
If the (C) line turns red and the (T) line turns black:
The test returned a positive result for the presence of coronavirus antigen.
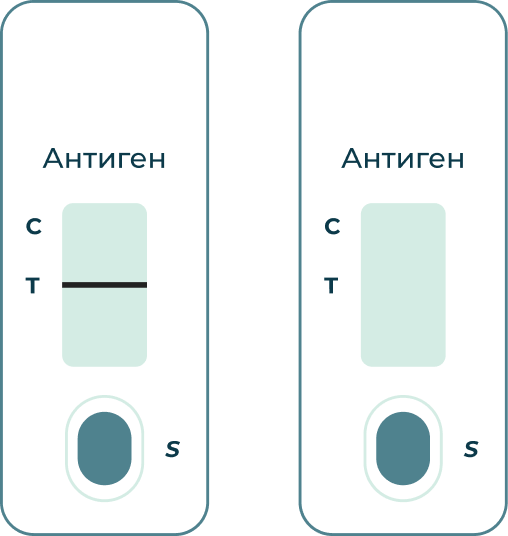
If the (C) line in the window does not turn red:
The test result is invalid. Possible causes could include non-compliance with biological sample collection requirements, and/or analysis procedures, or the test system used is faulty.